

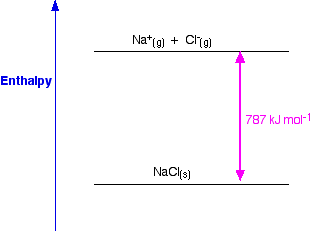
So, from the cycle we get the calculations directly underneath it. The diagram is set up to provide two different routes between the thick lines. Now we can use Hess' Law and find two different routes around the diagram which we can equate. And finally, we have the positive and negative gaseous ions that we can convert into the solid sodium chloride using the lattice formation enthalpy.Remember that first electron affinities go from gaseous atoms to gaseous singly charged negative ions. The -349 is the first electron affinity of chlorine.Again, we have to produce gaseous atoms so that we can use the next stage in the cycle. The +122 is the atomization enthalpy of chlorine.Remember that first ionization energies go from gaseous atoms to gaseous singly charged positive ions. The +496 is the first ionization energy of sodium.We have to produce gaseous atoms so that we can use the next stage in the cycle. The +107 is the atomization enthalpy of sodium.The Born-Haber cycle now imagines this formation of sodium chloride as happening in a whole set of small changes, most of which we know the enthalpy changes for - except, of course, for the lattice enthalpy that we want to calculate. The arrow pointing down from this to the lower thick line represents the enthalpy change of formation of sodium chloride. Notice that we only need half a mole of chlorine gas in order to end up with 1 mole of NaCl. We are starting here with the elements sodium and chlorine in their standard states. If you wanted to draw it for lattice dissociation enthalpy, the red arrow would be reversed - pointing upwards.įocus to start with on the higher of the two thicker horizontal lines. You will see that I have arbitrarily decided to draw this for lattice formation enthalpy. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others.\)Ĭonsider a Born-Haber cycle for sodium chloride, and then talk it through carefully afterwards.
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants.įrom providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation. Physics Wallah's main focus is to make the learning experience as economical as possible for all students. We believe in empowering every single student who couldn’t dream of a good career in engineering and medical field earlier. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We successfully provide students with intensive courses by India's top faculties and personal mentors. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app. We also provide extensive NCERT solutions, sample papers, NEET, JEE Mains, BITSAT previous year papers, which makes us a one-stop solution for all resources.

Physics Wallah is India's top online ed-tech platform that provides affordable and comprehensive learning experience to students of classes 6 to 12 and those preparing for JEE and NEET exams.


 0 kommentar(er)
0 kommentar(er)
